Predicting molecular self-assembly at surfaces: a statistical thermodynamics and modeling approach
Simone Conti, and Marco Cecchini
Physical Chemistry Chemical Physics, 2016, 18, pp 1480-31493.
DOI:10.1039/C6CP05249E | Find on RG
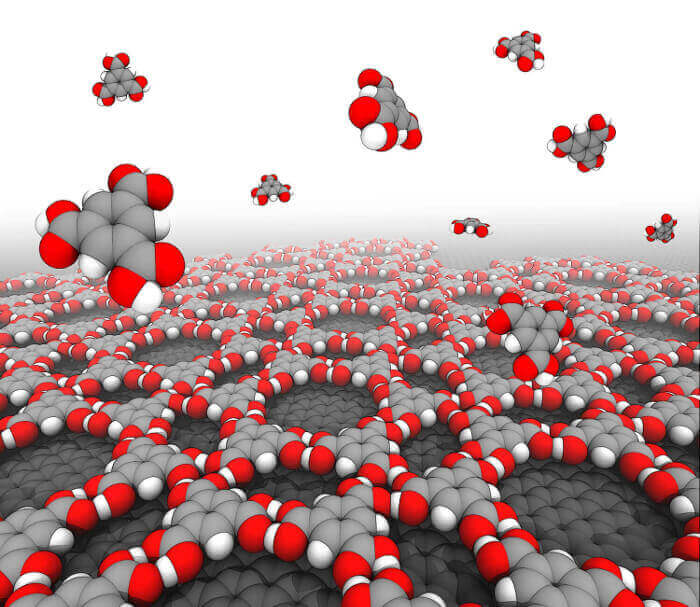
Abstract:
Molecular self-assembly at surfaces and interfaces is a prominent example of self-organization of matter with outstanding technological applications. The ability to predict the equilibrium structure of the self-assembled monolayer (SAM) is of fundamental importance and would boost the development of bottom-up strategies in a number of fields. Here, we present a self-consistent theory for a first-principle interpretation of 2D self-assembly based on modeling and statistical thermodynamics. Our development extends the treatment from finite-size to infinite supramolecular objects and delineates a general framework in which previous approaches can be recovered as particular cases. By proving the existence of a chemical potential per unit cell, we derive an expression for the surface free energy of the SAM (γ), which provides access to the thermodynamic stability of the monolayer in the limit of the ideal gas approximation and the model of energetics in use. Further manipulations of this result provide another expression of γ, which makes the concentration dependence as well as the temperature dependence of 2D self-assembly explicit. In the limit of the approximations above, this second result was used to analyze competitive equilibria at surfaces and rationalize the concentration-and temperature-dependent polymorphism in 2D. Finally, the theory predicts that there exists a critical aggregation concentration (Ccac) of monomers above which 2D self-assembly can be viewed as a "precipitation" in a solubility equilibrium. Numerical analysis of thirteen model SAMs on graphene shows that the value of Ccac sets an absolute scale of 2D self-assembly propensity, which is useful to compare chemically distinct and apparently unrelated self-assembly reactions.
