Rhodochrosite is not a commonly fluorescent mineral. Chemically and structurally, rhodochrosite is similar to the much more common calcite. The main difference is that while calcite is calcium carbonate, CaCO3, rhodochrosite is manganese carbonate, MnCO3. What makes the fluorescence problem interesting in rhodochrosite is that manganese impurities is what causes the typical red fluorescence in calcite. So, why rhodochrosite is not usually fluorescent if it contains only manganese? The red fluorescence in calcite is actually a quite complicated process. Manganese alone is not enough since, by itself, it is not able to interact with the UV light. It needs a co-activator, like lead, Pb2+, or cerium, Ce3+, which can interact with the UV light and transfer the energy to a nearby manganese ion. Too much manganese is also as issue, since it can act as a quencher or the fluorescence. Essentially, the energy from the UV light is not re-emitted as red light, but dissipated and lost if too many manganese ions are close together. And here we can see how the fluorescence in rhodochrosite is uncommon. Rhodochrosite is, by definition, very rich in manganese, making the self-quenching much more likely.
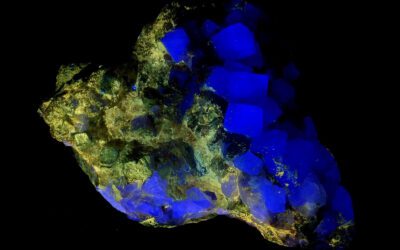
So, is fluorescence in rhodochrosite possible? Yes, it is. This is the only example in my collection, a nice pink rhodochrosite plate from the N’Chwaning II Mine in Northern Cape, South Africa. It fluoresces red under both longwave and midwave UV light, and dimmer under midwave UV.
Fluorescent rhodochrosite in my collection:
SC1117 – Rhodochrosite from the N’Chwaning II Mine, South Africa
Little plate of pink rhodochrosite crystals, all packed together. Contrary to every guess, the...