Molecular insights into the stabilization of protein–protein interactions with small molecule: The FKBP12–rapamycin–FRB case study
Shilpi Chaurasia, Stefano Pieraccini, Riccardo De Gonda, Simone Conti, and Maurizio Sironi
Chemical Physics Letters, 2013, 587, pp 68–74.
DOI:10.1016/j.cplett.2013.09.042 | Find on RG
Abstract:
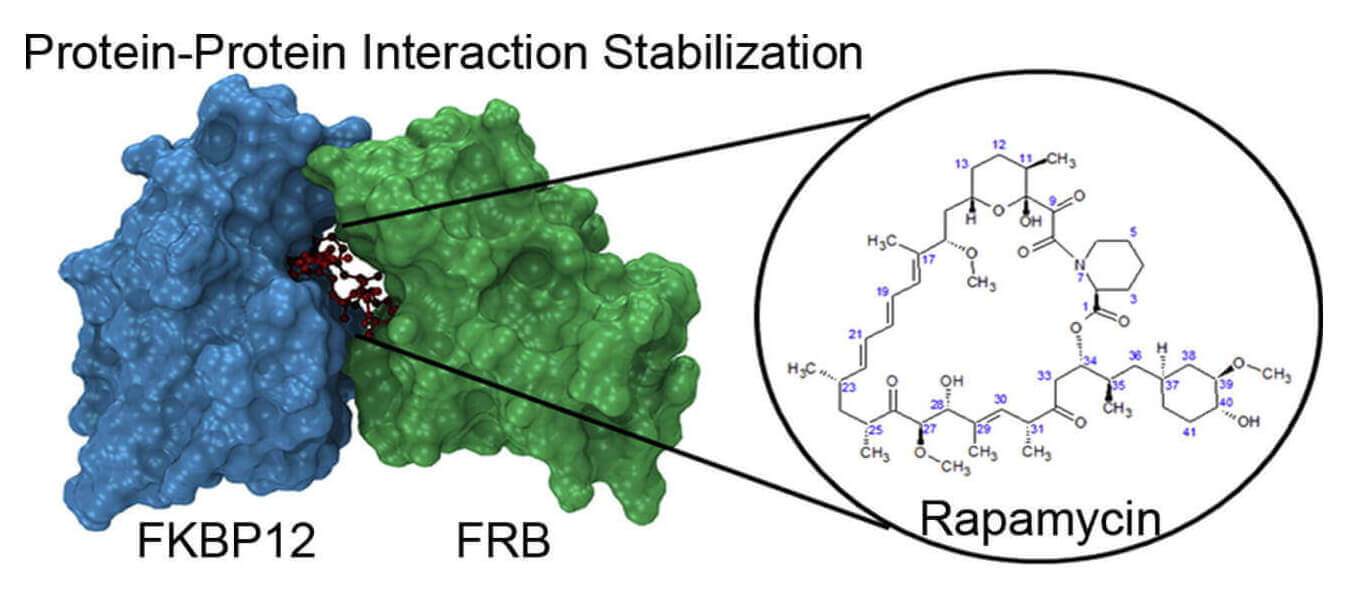
Targetting protein–protein interactions is a challenging task in drug discovery process. Despite the challenges, several studies provided evidences for the development of small molecules modulating protein–protein interactions. Here we consider a typical case of protein–protein interaction stabilization: the complex between FKBP12 and FRB with rapamycin. We have analyzed the stability of the complex and characterized its interactions at the atomic level by performing free energy calculations and computational alanine scanning. It is shown that rapamycin stabilizes the complex by acting as a bridge between the two proteins; and the complex is stable only in the presence of rapamycin.
